|
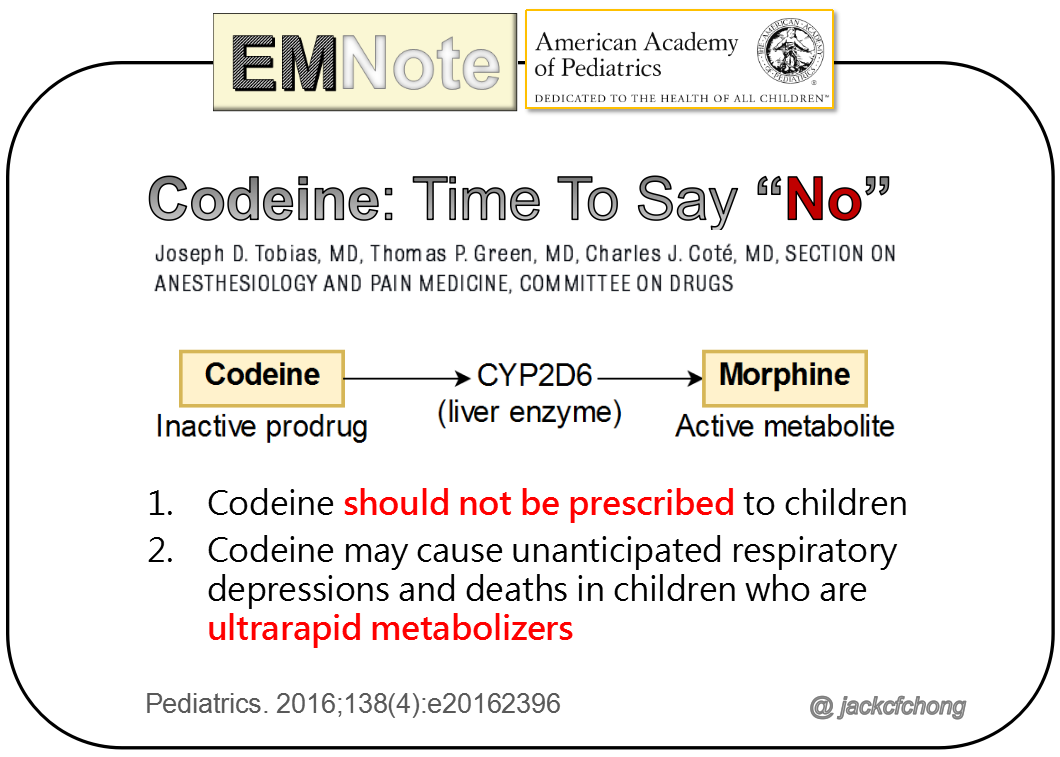
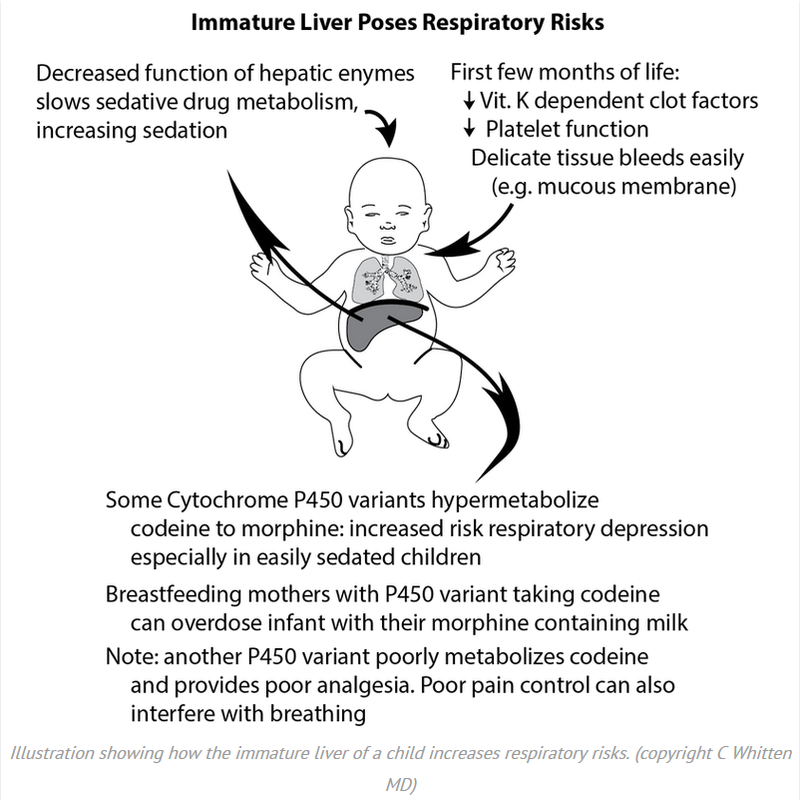
Codeine: Time To Say “No” Codeine is a prodrug with little inherent pharmacologic activity and must be metabolized in the liver into morphine, which is responsible for codeine’s analgesic effects. However, there is substantial genetic variability in the activity of the responsible hepatic enzyme, CYP2D6, and, as a consequence, individual patient response to codeine varies from no effect to high sensitivity. Drug surveillance has documented the occurrence of unanticipated respiratory depression and death after receiving codeine in children, many of whom have been shown to be ultrarapid metabolizers. Patients with documented or suspected obstructive sleep apnea appear to be at particular risk because of opioid sensitivity, compounding the danger among rapid metabolizers in this group. Recently, various organizations and regulatory bodies, including the World Health Organization, the US Food and Drug Administration, and the European Medicines Agency, have promulgated stern warnings regarding the occurrence of adverse effects of codeine in children. These and other groups have or are considering a declaration of a contraindication for the use of codeine for children as either an analgesic or an antitussive. Pediatrics. 2016;138(4):e20162396 Comments are closed.
|

Author
|
Proudly powered by Weebly